Research Project 6:
Cell type-specific roles of chloride/proton exchange in lysosomes
Principal Investigators
Prof. Dr. rer. nat. Tobias Stauber
Prof. Dr. rer. nat. Dr. med. Thomas J. Jentsch
PhD Students (FOR2625 funded)
Mariia Zeziulia, MSc
associated co-workers
Dr. rer. nat. Malte Klüssendorf
Dr. Maya Polovitskaya

Project Summary
ClC-7 is a Cl-/H+ exchanger that, together with its beta-subunit Ostm1, localizes to lysosomes and to the osteoclast ruffled border. Dysfunction of ClC-7/Ostm1 leads to osteopetrosis and a neurodegenerative lysosomal storage disease in mice and patients. The osteopetrosis may in part be caused by the underdevelopment of the ruffled border of osteoclasts, which is normally formed by lysosomal exocytosis. The lysosomal dysfunction, which involves a slowed protein degradation and the accumulation of autophagic material, is likely due to alterations of the lysosomal ion composition. Using cells derived from our various mouse models with different mutations in the Clcn7 gene, we found that lysosomes were acidified to a normally low pH. However, we found a drastic decrease in the lysosomal Cl- concentration upon ClC-7 deficiency or its conversion into a pure Cl- channel. Different ClC-7 variants in patients and mouse models result in diverse phenotypes with bones and extraskeletal tissues affected to varying degrees, suggesting cell type-specific roles for ClC-7. This project addresses these cell type-specific functions and the involvement of ClC-7 in lysosomal ion homeostasis in general. One focus lies on the differential roles of ClC-7 in bone resorption and basic lysosomal function. We will study and compare the effects of various ClC-7 mutations on the ion concentrations, morphology and function of lysosomes in different cell types, including neurons and osteoclasts, from our respective mouse models. Another aspect is the function of ClC-7 in the immune system which we will study using cell type-specific ClC-7 KO mice. Lastly, we plan to gain mechanistic insight into the role of ClC-7 by measuring its activity and its impact on various parameters of lysosomal ion homeostasis.
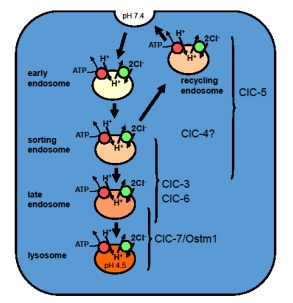
Schematic diagram of CLC transporters in the endosomal/lysosomal pathway. These intracellular 2Cl–/H+ exchangers that operate in parallel to the H+-ATPase and influence luminal Cl– and H+ concentrations. The Jentsch lab has generated mouse models for vesicular CLCs and mutations in CLCN4, -5, -6, and -7 underlie various human genetic diseases.
References
Weinert S, Gimber N, Deuschel D, Stuhlmann T, Puchkov D, Farsi Z, Ludwig CF, Novarino G, López-Cayuqueo KI, Planells-Cases R, Jentsch TJ. Uncoupling endosomal CLC chloride/proton exchange causes severe neurodegeneration. EMBO J 2020; 39: e103358.
Astaburuaga R, Quintanar Haro OD, Stauber T*, Relógio A*. A mathematical model of lysosomal ion homeostasis points to differential effects of Cl– transport in Ca2+ Cells. 2019; 8:E1263. (*corresponding authors)
Leisle L, Ludwig CF, Wagner FA, Jentsch TJ*, Stauber T. ClC-7 is a slowly voltage-gated 2Cl–/1H+-exchanger and requires Ostm1 for transport activity. EMBO J. 2011;30: 2140-52. (*corresponding author)
Weinert S, Jabs S, Supanchart C, Schweizer M, Gimber N, Richter M, Rademann J, Stauber T, Kornak U, Jentsch TJ. Lysosomal pathology and osteopetrosis upon loss of H+-driven lysosomal Cl– Science 2010; 328:1401-3.
