Research Project 9:
Understanding the role of the Lysosomal Integral Membrane Protein type 2 (LIMP-2/SCARB2) in lysosomal lipid transport
Principal Investigator
Prof. Dr. rer. nat. Paul Saftig
Postdoctoral Fellow (FOR2625 funded)
Dr. rer. nat. Sönke Rudnik

Project Summary
Lipid accumulation in lysosomes is a hallmark of lipidoses, which can lead to a number of lysosomal storage disorders such as Niemann-Pick disease, Sandhoff disease, Tay-Sachs disease, Farber disease, and Gaucher’s disease, depending on the type of accumulated lipid. Recently, we provided evidence that the abundant lysosomal membrane protein LIMP-2/SCARB2 contributes to the recycling of lysosomal LDL-derived cholesterol alongside the NPC1 pathway. We demonstrated that LIMP-2, which is belonging to the CD36 superfamily of scavenger receptors, directly interacts with cholesterol and can transport it to the limiting membrane via the LIMP-2 ectodomain tunnel. Since LIMP-2 also plays a role in the transport of -glucocerebrosidase to lysosomes and is involved in phospholipid transfer events the further elucidation of its molecular role in lysosomal lipid transport processes is required. Based on our recently published work and our preliminary data we hypothesize that LIMP-2 serves as a sterol (lipid) transport protein at lysosome-endoplasmic reticulum (ER) membrane contact sites. Within the proposed project we will test this hypothesis by biochemically analyzing additional lipid substrates which utilize the LIMP-2 tunnel pathway. In preliminary studies using co-immunoprecipitation screens we identified endosomal and ER-resident membrane proteins as LIMP-2 interactors. We plan to further analyze their interaction and their role in the formation of membrane contact sites using immunoprecipitation and microscopy localization experiments. Overexpression and loss of expression analyses will allow us to decipher the importance of such LIMP-2 complexes for lipid transport. We will also study the role of palmitoylation of LIMP-2 to regulate such membrane contact site formation and lipid transport processes. In summary, within the framework of the research unit our project will add valuable insight in how LIMP-2 exerts fundamental roles in transferring lipids from the lysosomal lumen to the limiting membrane and to other cellular compartments.
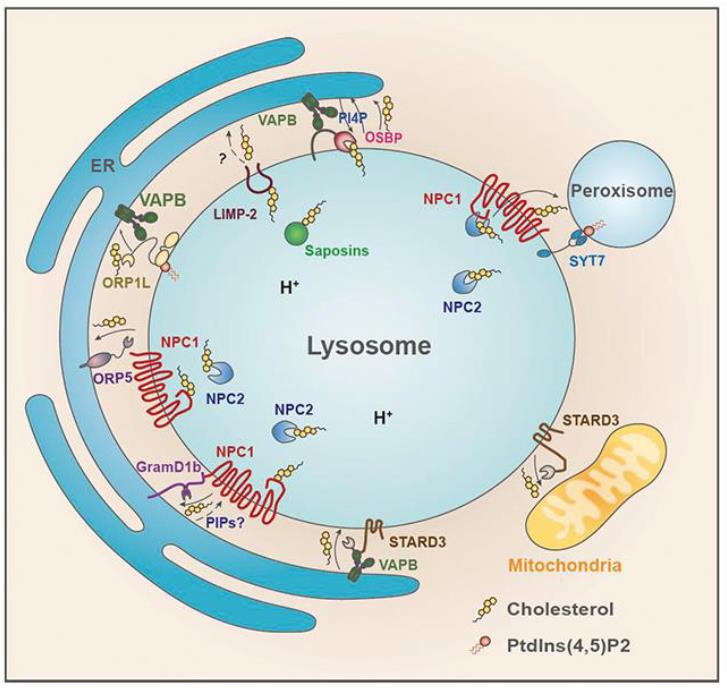
Lysosome-organelle contact sites mediated by NPC1, STARD3 and possibly LIMP-2, respectively. Image from (Meng, Heybrock, Neculai, Saftig. Trends Cell Biol. 2020).
References
Meng Y, Heybrock S, Neculai D, Saftig P. Cholesterol handling in lysosomes and beyond. Trends Cell Biol 2020; doi:1016/j.tcb.2020.02.007.
Heybrock S, Kanerva K, Meng Y, Ing C, Liang A, Xiong ZJ, Wenig X, Kim YA, Collins RA, Trimble W, Pomès R, Privé GG, Annaert W, Schwake M, Heeren J, Lüllmann-Rauch R, Grinstein S, Ikonen E, Saftig P, Neculai D. Lysosomal Integral Membrane Protein-2 (LIMP-2/SCARB2) is involved in lysosomal cholesterol export. Nat Commun 2019; 10:3521.
Neculai D, Schwake M, Ravichandran M, Zunke F, Collins RF, Peters J, Neculai M, Plumb J, Loppnau P, Pizarro JC, Seitova A, Trimble WS, Saftig P, Grinstein S, Dhe-Paganon S. Structure of LIMP-2 provides functional insights with implications for SR-BI and CD36. Nature 2013; 504:172-6.
